Share this Page:
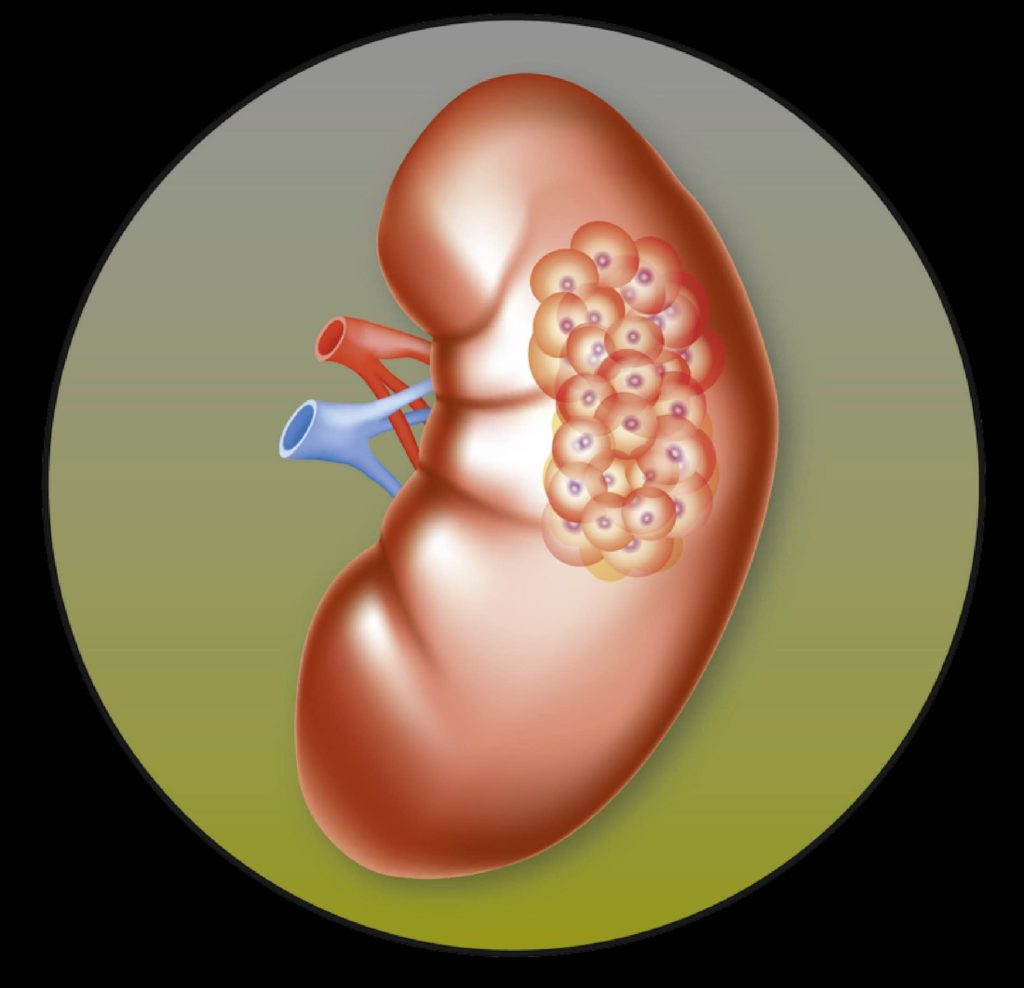
This week saw the initiation of a global phase III clinical trial of lenvatinib, a multiple receptor tyrosine kinase inhibitor, in combination with everolimus and the anti-PD-1 antibody, pembrolizumab, as a potential first-line treatment for advanced renal cell carcinoma (RCC). The trial is called the CLEAR study, and is being run by the Japanese pharmaceutical company, Eisai. The first sites have been initiated in America and the company expect to open sites in the UK in Spring 2017.
The CLEAR study is a multicentre, randomised, open-label phase III clinical study to compare the efficacy and safety of lenvatinib/everolimus and lenvatinib/pembrolizumab versus sunitinib alone as a first-line treatment for people with advanced RCC. The primary outcome measure will be progression-free survival.